Continuous Inline Size Monitoring Low Turbidity Suspensions under Flow in Biopharmaceutical Processes
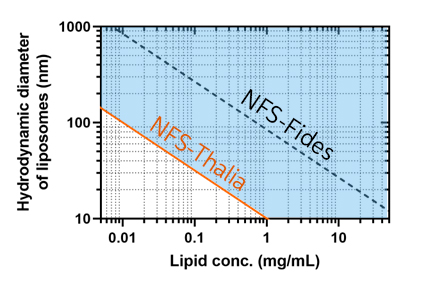
The challenges in manufacturing biopharmaceuticals involve precise control and monitoring of processes, hindered by traditional methods like Dynamic Light Scattering (DLS) unable to offer continuous inline measurements. This leads to inefficient formulation development and delays in production approval, impacting supply chains and responsiveness to demand fluctuations. To address these issues, Quality by Design (QbD) principles advocate for intentional quality design through Process Analytical Tools (PAT), such as the innovative ‘Spatially Resolved Dynamic Light Scattering’ (SR-DLS) technology developed by InProcess-LSP. This advanced instrumentation, exemplified by the NFS-Thalia system, enhances nanoparticle sizing capabilities, even in low turbidity formulations, enabling real-time monitoring and improving process efficiency and quality.
This application note focuses on the use of SR-DLS technology for the continuous size monitoring of liposome manufacturing at low concentrations and sub-10 nm protein measurement using the newly developed high-sensitivity NFS-Thalia system.
Read the full whitepaper:


Up to millions is lost per year due to the absence of appropriate
particle size monitoring solutions…