How PAT can provide you cost reduction
Why do you first need to know about PAT
What is PAT?
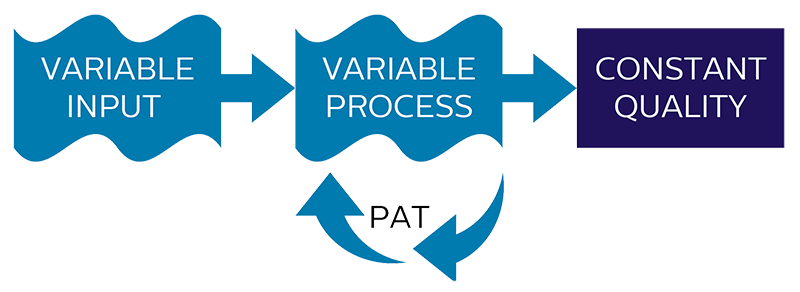
When timing in a dynamic process is crucial assuring target specifications in a consistent and reliable way, real-time process control becomes essential. Since input variables like raw material properties, process and environmental conditions will vary for most processes, the end-product of the process will consequently show related variations in quality as well.
Real-time process feedback
If a process is designed as a fixed process, without the possibility to adjust or cope with varying input variables, process performance will be dependent on how strict critical input variables can be controlled and how well the desired end-point of the process can be obtained without real-time feedback of the process itself.

Critical process parameters
By applying PAT to measure critical process parameters, processes can be made variable by adjusting the process for variations in input variables and detection of the desired end-point of the process.
Once the relation between critical input variables is known and adjusted for by the variable process, a consistent end-product quality and a higher level of process performance will be obtained.
Quality risks of your process
Typically for Pharma, Health agencies and regulatory bodies like FDA and EMA are not surprisingly interested in quality risks of process and product-related variations in relation to input variables.
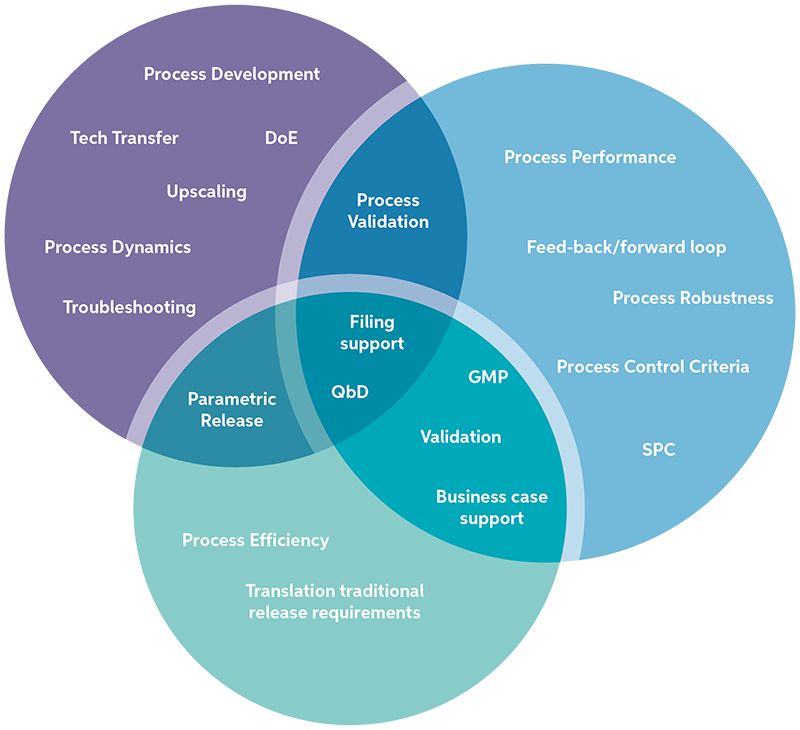
PAT will help to increase understanding, efficiency and control of your processes and product during development, upscaling and commercial production.
FDA Guidance
Besides process control, PAT data can be used efficiently to increase process understanding during development, validation, transfer or routine manufacturing. PAT data can be seen as a process signature allowing for in-depth interpretation, mechanistic understanding and continuous improvement of your process.
Real-Time Release
A third possibility is to employ PAT as a substitute for traditional release testing, known as Real-Time Release. This approach saves laborious analytical testing and logistical efforts and shortens the cycle time of manufacturing significantly.
Besides direct benefits from a process quality en performance perspective, health agencies like FDA strongly encourage the use of PAT and available technologies to improve process understanding and performance:
4 reasons to apply PAT
Efficiency
Elimination of sampling Inline analysis Shorter production time Shorter development time
Understanding
Process dynamics Critical process parameters Continuous improvement
Risk of failure
Batch rejection Sterility issues Representative sample
Opportunity

Precise manufacturing Quality control